Water vapor absorbs and reradiates more of the EM signal in UHF, VHF and EHF frequencies than other types of molecules in the troposphere. This is because the molecule is polar - this means that the electrons tend to stay on one side of the molecule as shown in the figure, but its polar orientation also flips, moving the electrons a larger distance. This is why water vapor has a relatively large effect on refraction for these frequencies.
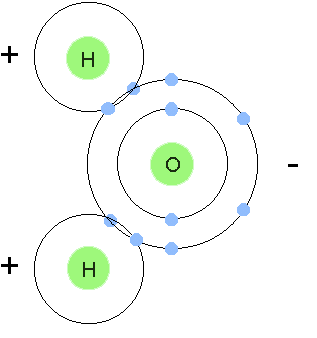 |
H2O, the water molecule. Note that the electrons, with their negative charges, are shared between the hydrogen atoms and the oxygen atom, creating a covalent bond, which leads to the electrons spending more time on one side of the molecule, giving it opposite charges on opposite ends. Hence the term "polarized". |